Abstract
Introduction
The identification of somatic genomic alterations in cancer has brought forth a paradigm change in the personalized management of patients with acute myeloid leukemia (AML). This is exemplified by the recent FDA approvals with the targeted small molecule inhibitors of mutant IDH1 (ivosidenib) and IDH2 (enasidenib) for patients with relapsed/refractory AML. IDH1/2 mutations have been reported to occur in approximately 16-20% of patients with AML based on the largest published cohorts to date (Dinardo et al., Am J Hematol, 2015; Papaemmanuil et al., NEJM, 2016). However, there remains a paucity of data on the frequency of IDH1/2 and other targetable mutations in extramedullary manifestations of AML (i.e. myeloid sarcoma and leukemia cutis; EM-AML). We sought to characterize the mutational landscape of EM-AML, with a focus on determining the frequency of IDH1/2 mutations.
Methods
This is a multi-institutional retrospective analysis of patients diagnosed with EM-AML, who were treated at Moffitt Cancer Center or Memorial Healthcare System and underwent next-generation sequencing (NGS). All patients were evaluated for IDH1/2 mutations and up to 435 additional genes. Clinical variables and outcomes of EM-AML patients were characterized at the time of sample procurement. Additionally, we acquired additional de-identified NGS data on EM-AML patients who underwent sequencing of the EM site at Genoptix. We describe the mutational landscape of patients and compared the frequency of IDH1/2 mutations to historical controls in AML from the published literature using Fisher's exact test. Kaplan-Meier curves were used to estimate overall survival (OS) and analyzed from the date of mutation identification.
Results
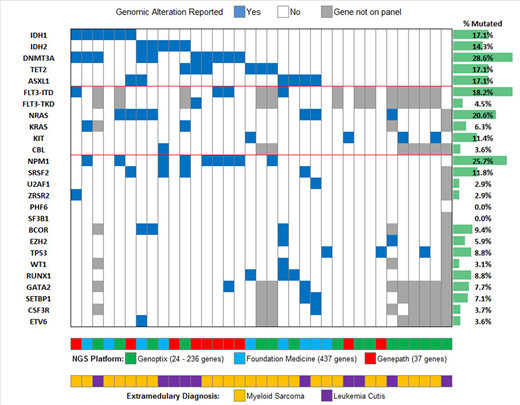
Thirty-five patients with EM-AML were identified (22 in the clinical cohort and 13 in the Genoptix cohort) and are included in this analysis. The distribution of EM-AML diagnoses were myeloid sarcoma in 71% of cases (n=25) and leukemia cutis in 29% (n=10). The sequenced samples were from an extramedullary site for 63% (n=22) of the cases. The median age of the cohort was 64 (IQR 52 - 72) with a male predominance (55%). Of the clinical cohort, 68% (n=15) were non-Hispanic White, 23% (n=5) were Hispanic, and 9% (n=2) were Black. The molecular landscape of the cohort is shown in Figure 1. The frequency of IDH1/2 mutations was 31% (11/35), with IDH1 and IDH2 mutations reported in 17% (n=6) and 14% (n=5) of cases, respectively. All IDH1 mutations were at position R132 (4 R132H and 2 R132C) and all IDH2 mutations were at R140 (4 R140Q and 1 R140G). The other most frequent genomic alterations were DNMT3A (29%), NPM1 (26%), FLT3 (23%; ITD in 18% and TKD in 5%; 11% in IDH wildtype), NRAS (21%), TET2 (17%), and ASXL1 (17%). Mutations in DNA methylation occurred in 54% of patients (n=19). Co-occurring NRAS/KRAS mutations were more frequent in IDH mutant patients (45%; 5/11) compared to 12% (3/24) of IDH wild type patients (P=0.01). DNMT3A mutations were reported in 45% (5/11) of IDH mutant cases compared to 21% (5/24) of IDH wildtype cases, although this was not statistically significant (P=0.23). In comparison to a historical frequency of IDH1/2 mutations in AML patients of 17.4% (n=2366), EM-AML patients had an increased frequency of 31% (P=0.04). The median OS of the cohort was 13.6 months. Within the clinical cohort 50% (4/8) of the IDH1/2 mutant patients were treated with an IDH inhibitor with CR in 1 patient, CRi in 1 patient, and stable disease in 2 patients. The patient who achieved CR developed widespread leukemia cutis during cycle 1 of enasidenib which resolved over 6 months and is in durable CR for 15 months.
Conclusion
Overall, EM-AML patients had targetable mutations (i.e. IDH1/IDH2/FLT3) in 40% of cases with an increased frequency of IDH1/2 mutations (31%) in comparison to the general AML population. IDH mutant EM-AML patients achieved clinical benefit to specific inhibitors. These data support mutational analysis of EM-AML patients in order to personalize therapeutic options for our patients.
Bhagat:Genoptix: Employment. Watts:Takeda: Research Funding; Jazz Pharma: Consultancy, Speakers Bureau. Sweet:Novartis: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Jazz: Speakers Bureau; Celgene: Honoraria, Speakers Bureau; Jazz: Speakers Bureau; BMS: Honoraria; Astellas: Consultancy; Celgene: Honoraria, Speakers Bureau; Astellas: Consultancy; Agios: Consultancy; Agios: Consultancy; Phizer: Consultancy; Phizer: Consultancy; BMS: Honoraria. Komrokji:Celgene: Honoraria, Research Funding; Novartis: Honoraria, Speakers Bureau; Celgene: Honoraria, Research Funding; Novartis: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau. Sallman:Celgene: Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal